INTRODUCTION
VTE is the second leading cause of death in cancer patients, and is associated with a 47-fold increased risk of mortality (Khorana et al. 2007). While complications such as death and pulmonary embolism are less common in cases of upper extremity deep venous thrombosis (UEDVT) and central venous catheter (CVC) associated UEDVT compared to lower extremity deep venous thrombosis (DVT), they still represent a clinically significant problem. A systematic review determined that the risk of recurrent VTE in cancer-associated UEDVT was as high as 9.8% and the risk of major bleeding during anticoagulation was as high as 6.7% (Bleker et al. 2016).
Central venous catheter placement is universally employed in individuals undergoing hematopoietic stem cell transplantation (HSCT), where the incidence of CVC-associated VTE has been reported at 3.6% (Gerber et al. 2008). The post-HSCT setting is characterized by multiple risk factors for both thrombosis and hemorrhage, including a high incidence of thrombocytopenia. Efforts to reduce the incidence of post-transplant VTE with prophylactic anticoagulants have been met with inconclusive outcomes (Akl et al. 2007). Prospective studies of treatment after the diagnosis of CVC-associated UEDVT are lacking, and evidence-based guidelines are derived largely from studies of lower-extremity DVTs. In the real world, anticoagulation is often held entirely when a clinician has concern regarding a patient's risk for bleeding and during refractory thrombocytopenia. The clinical significance of post-transplant CVC-associated UEDVT remains unclear, and a better understanding may help to guide treatment in this highly complex patient population.
METHODS
To investigate the clinical impact of CVC-associated UEDVT in the post-HSCT setting, we performed a retrospective chart review of individuals at the University of Colorado Hospital who underwent allogeneic stem cell transplantation for non-myeloma hematologic malignancies between January 2010 and March 2016. Two-hundred and fifty-five patient charts were reviewed. Fifty-eight patients who developed radiographically confirmed extremity VTE within the first year after transplant were further analyzed with regards to re-thrombosis and bleeding outcomes up to 6 months post-VTE. Patients who had CVC-associated UEDVT were compared with those who had non-CVC-associated VTE. Patients with CVC-associated UEDVT were further broken down into two groups based on whether they received anticoagulation after initial VTE diagnosis. Descriptive statistics and Fisher exact tests were used to compare these groups.
RESULTS
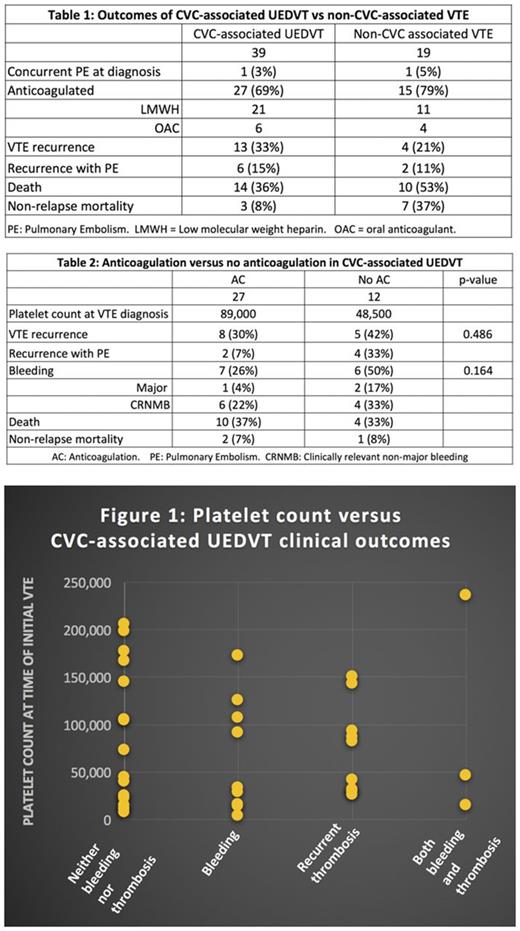
Results are presented in Tables 1 and 2. Compared to the non-CVC-associated VTE group, individuals with CVC-associated UEDVT had a lower non-relapse mortality (8% vs 37%) despite receiving less therapeutic anticoagulation. In the CVC-associated VTE group, patients who did not receive anticoagulation had a lower average platelet count at the time of initial VTE diagnosis, and there was no significant difference in the recurrence of VTE (30% vs 42%, p = 0.486). While there was not a statistically significant difference in bleeding, there did appear to be a trend for increased overall bleeding in the patients with CVC-associated UEDVT who were not anticoagulated (overall: 26% vs 50%, p = 0.164; clinically relevant non-major bleeding: 22% vs 33%; and major bleeding: 4% vs 17%). The relationship between platelet count and clinical outcomes of CVC-associated UEDVT is presented in Figure 1.
CONCLUSIONS
This study continues to demonstrate the high risk for thrombosis and bleeding in individuals in the post-HSCT setting. Neither receipt of anticoagulation nor platelet count appears to correlate with the risk for recurrent VTE or adverse bleeding outcomes in individuals with CVC-associated UEDVT. Although this is retrospective data inclusive of small numbers of events, these outcomes should be considered by physicians whose clinical judgement warns against therapeutic anticoagulation for an individual patient in this setting. The results may also serve to caution investigators considering additional study on CVC-associated VTE prophylaxis during HSCT. Further research is needed to aid clinicians in risk stratification of this highly complex patient population, such that prophylaxis and treatment recommendations can be more confidently prescribed.
Buckner: CSL Behring: Membership on an entity's Board of Directors or advisory committees; Uniqure: Consultancy; Shire: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal